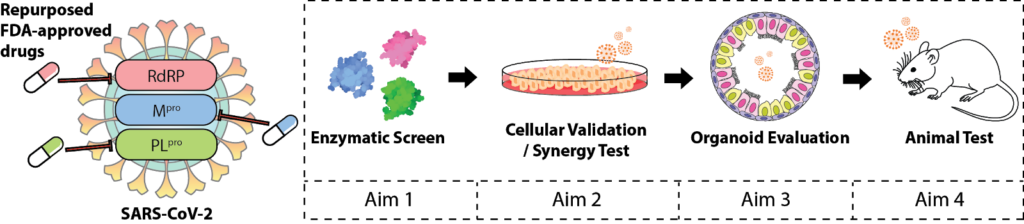
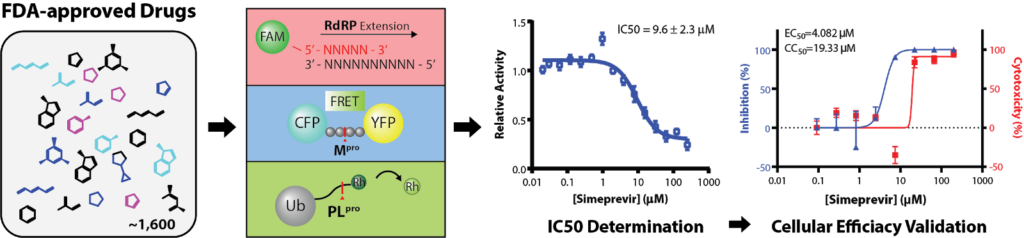
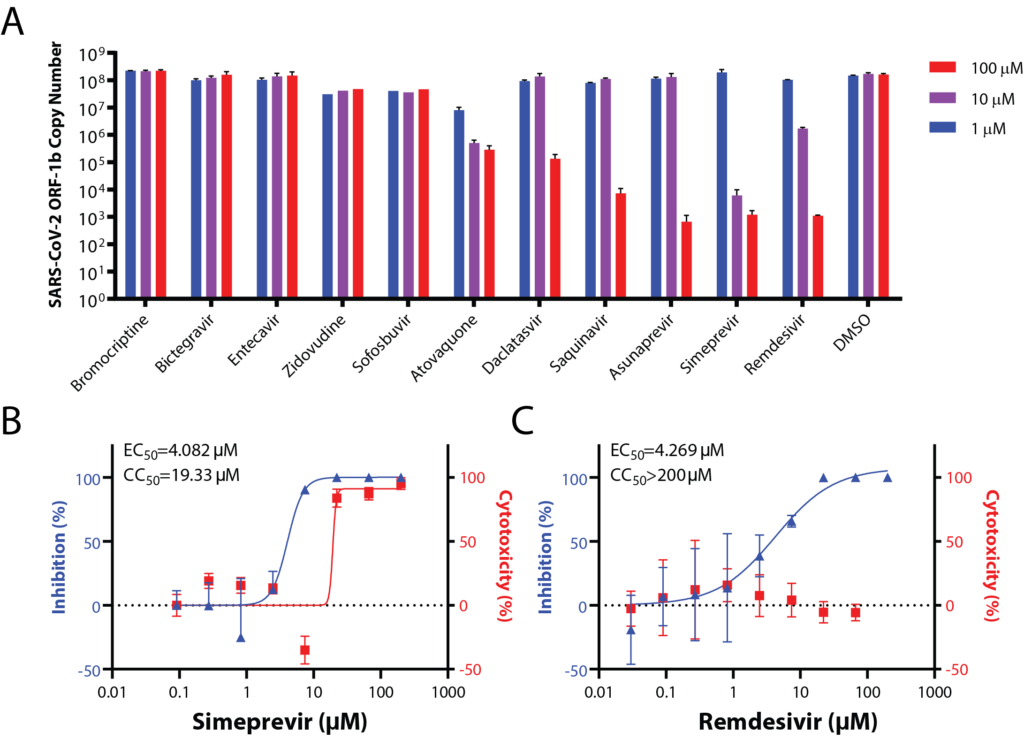
The recent outbreak of coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, is a global threat to human health. However, there are currently no drugs for treating this devastating disease. The existing drug, remdesivir, has only one known protein target and is prone to drug resistance. Using in vitro screening and biochemical characterization, we identified the several FDA-approved drugs, such as the hepatitis C virus (HCV) protease inhibitor Simeprevir, as promising candidates for treating COVID-19. Simeprevir exhibits a similar suppression efficacy against SARS-CoV-2 viral replication as remdesivir and synergizes with remdesivir in vitro which lowers effective dose. Mechanistically, we showed that simeprevir inhibits both of the main protease (Mpro) and RNA-dependent RNA polymerase (RdRP) and this drug has known pharmacokinetics. Our results thus reveal unexpected viral protein targets of this drug and provide preclinical rationale for the combination of FDA-approved drugs and remdesivir for the pharmacological management of COVID-19 patients.